The University of Queensland's Clinical Trials Centre (UQ CTC) is a specialised centre that collaborates with health and medical researchers, clinicians and other partners in the design, conduct, analysis, publication, and translation of randomised clinical trials (RCTs).
Our collaborative approach
The UQ CTC is an interdisciplinary research development and delivery team with highly skilled members who have extensive experience in large-scale clinical trials. Supported by a comprehensive Quality Management System and associated Standard Operating Procedures the CTC provides end-to-end collaboration on high-quality, UQ-sponsored, UQ investigator-led, RCTs that improve the health and well-being of Australians and beyond.
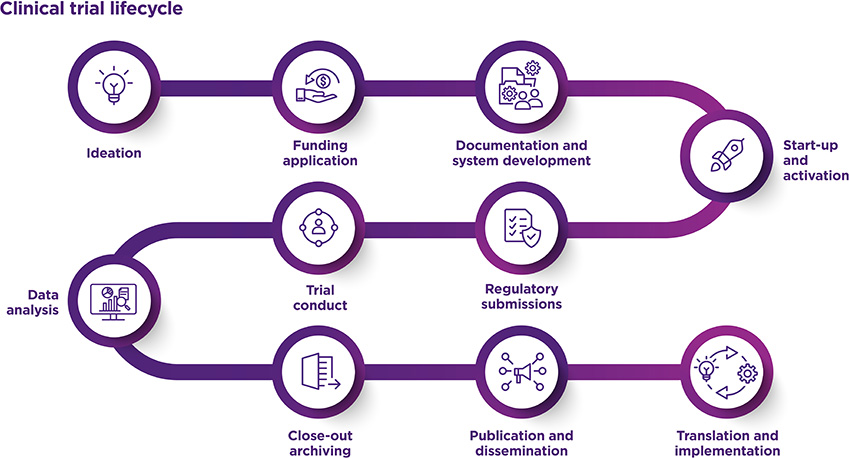
We collaborate with UQ Chief Investigators and their research teams throughout the entire clinical trial lifecycle, starting from the initial idea. We assist with funding applications, protocol development, and trial conduct through to data analysis, sharing of results and implementation and translation.
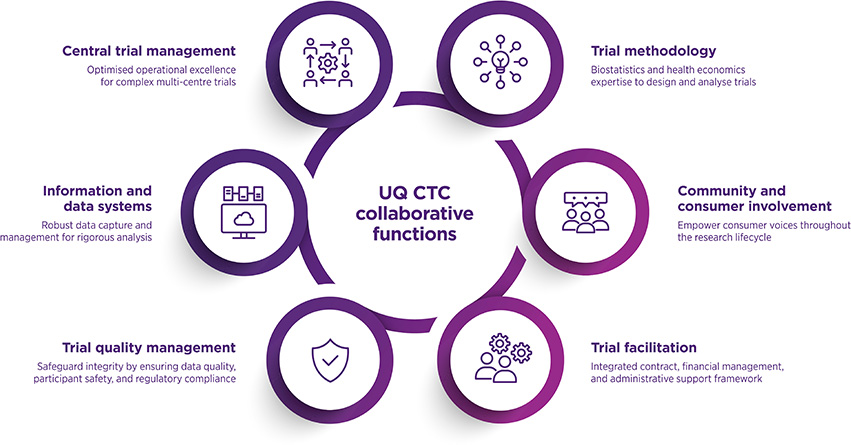
Our core capabilities include central trial management, database development and data management, statistics, trial quality assurance, and support for Consumer and Community Involvement (CCI).
A core requirement of collaboration with the CTC is that central trial management and statistical functions are delivered by the CTC team. Additional CTC collaborative functions are agreed through consultation to address each trial's unique requirements and complexities.
We work together with ULTRA, a highly skilled interdisciplinary team focused on creating innovation in RCTs, to augment existing expertise and accelerate innovative RCT capability, capacity, co-ordination and competitiveness.
Leadership and governance
The Centre is led by Professor Nadine Foster, Academic Director; and Dr Roberta Littleford, Associate Director and Head of Operations.
Strategic guidance from a state and national perspective is provided by the UQ CTC Advisory Board. The Board comprises an independent Chair, with representatives from other major clinical trial centres in Australia, Queensland Health, Health Translation Queensland, a lead health consumer advocate and UQ Executive Deans.
The diverse RCT experience and expertise of members of the UQ CTC Leadership Group is informing its design and operational rollout during the CTC's establishment phase. The UQ CTC Adoption Group, a subset of the Leadership Group, plays a crucial role in determining which trials the UQ CTC collaborates with.
| |
Nadine is an experienced clinical trialist, passionate about leading interdisciplinary teams that bring together the critical mass needed to design and conduct high quality randomised trials that impact on health and well-being. Her experience includes evaluations of conservative care interventions such as exercise and rehabilitation, combined physical and psychological therapies, surgery and new models of healthcare such as stepped care and stratified care. She joined The University of Queensland in 2020 and was appointed Academic Director of UQ Clinical Trials Centre in 2023. Nadine is also the program lead for the Health Research Accelerator (HERA) program ULTRA which means UQ’s Clinical Trials Capability. The CTC and ULTRA teams work in an integrated way to support clinical trial capability and capacity, co-ordination and competitiveness at UQ. In UQ CTC, Nadine provides academic leadership and research direction to the interdisciplinary team of CTC staff, ensures the CTC collaborates on a broad portfolio of clinical trials, and designs and conducts methodological research to improve how trials are conducted. With other academic staff Nadine is responsible for designing and conducting high quality clinical trials, mentoring and supporting the next generation of clinical trialists. | |
 Dr Roberta LittlefordAssociate Director and Head of Operations | |
Roberta has extensive clinical research experience and combines a unique blend of clinical, academic, project, and strategic leadership. Through multidisciplinary national and international collaborations, Roberta has delivered a portfolio of clinical trials involving investigational products and complex interventions, across all clinical trial phases. She is particularly proud to have been central to three flagship research projects on lung cancer and cardiovascular screening modalities and personalised asthma treatment for children and young adults. She joined The University of Queensland in 2018 and was appointed Associate Director and Head of Operations of UQ CTC in 2023. Prior to her return to Australia, Roberta was the Strategic Director of a UK Registered Clinical Trials Unit, providing leadership in the development and delivery of best practice, policy, and compliance improvement strategies. In the UQ CTC, Roberta leads organisational excellence and strategic goal achievement, ensuring the CTC meets the highest quality standards. By championing a collaborative, One-UQ approach, Roberta fosters and guides a high-performing, multidisciplinary team, nurturing talent and advancing skills, promoting strong infrastructure and core trial functions to promote a centre of excellence for clinical research, leading to better health and wellness outcomes for Queenslanders and beyond. | |
 Mark ChatfieldSenior Statistician | |
Mark is an experienced statistician and clinical trialist. Since 2002, he has been involved in numerous trials of various designs across a broad range of fields including: infectious disease, nutrition, respiratory disease, cerebral palsy and Indigenous health. He joined The University of Queensland in 2018 to collaborate with health and medical researchers. He plays an active role in the Australian Clinical Trials Alliance Statistics in Trials Interest Group. In the UQ CTC Mark provides statistical input into trial design, conduct, analysis, presentation and interpretation. | |
 Anna MorrisProject Manager | |
Anna has managed and administratively enabled the successful delivery of a range of strategic and complex programs, projects, operations, and initiatives applied across research, government, healthcare, and innovation contexts. She has helped support the initial build and establishment of new health and government services and thrives on supporting teams that explore turning ideas into impactful outcomes. In the UQ CTC Anna facilitates the establishment and operationalisation of the Centre to achieve its five-year strategic and operational goals, including design, implementation and continuous improvement of the Centre’s business systems, operations and processes. Anna manages projects and initiatives to deliver the CTC’s strategic goals, including procurement, communications, collaboration processes, and governance and reporting activities. |
Who we work with
The CTC is agnostic to population, condition, disease, or field of expertise, accommodating the breadth of the current UQ RCT portfolio and UQ's future aspirations.
We collaborate on multi-centre RCTs, although may consider collaboration on feasibility and pilot studies; single-centre RCTs; high-impact cohort studies; or other studies with a high probability of leading to a multi-centre trial, or the potential to have considerable impact on practice or policy.
For CTC collaborators, our team undertakes central trial management and statistical functions for the trial, along with other functions and activities as agreed. We work alongside your team in the ideation and proposal development stage, then as co-applicants, project team delivery members and co-authors, as appropriate. We work in partnership to establish cost recovery associated with the UQ CTC collaborative functions from the funders of the trial.
UQ staff members and Academic Title Holders can find out more information and submit a request to collaborate with the CTC (staff login required).
We also welcome strategic partnership opportunities that strengthen connections between researchers, the health system, clinical trial sites, and industry partners to help transform clinical trial excellence in Queensland. Email uqctc@uq.edu.au to discuss potential partnerships.


